What Makes Popcorn Pop Background Research
The Science of Popcorn

Mike Isley
Product Developer
Introduction
Popcorn is one of the world's favorite snack foods. In the US, Americans consume as much as 18 billion quarts of popcorn each year, which equates to 56 quarts per person. Some nutritionists telephone call information technology a perfect snack food considering information technology is a whole grain, a adept source of fiber, and low in fat. Ane study even claims there are more antioxidants in popcorn than in some fruits and vegetables.1
Groundwork
The nearly intriguing part of popcorn is the scientific discipline backside how it pops. Popcorn is the just grain in the corn family that pops open when exposed to temperatures above 180° C. A popcorn kernel is composed of three parts: the pericarp, germ, and endosperm. See Fig. 1.
The pericarp is the tough outer trounce surrounding a popcorn kernel, and the key to what makes it pop. Within the pericarp is the germ, or seed embryo. Next to the germ is the endosperm, which contains some trapped water plus soft and hard starch granules that serve equally food for the germ when information technology sprouts.
When a popcorn kernel is heated, the trapped water in the endosperm turns into steam, building up pressure level inside the pericarp. This pressurized, super-heated steam transforms the soft starch in the endosperm into a gelatinous fabric. Popcorn pericarp is much stronger than that of all other corn kernels and is able to retain this pressurized steam upward to 9.2 atm (135 psi).
Above that pressure, the pericarp ruptures, releasing the steam and gelled starch that solidifies upon cooling. The resulting popped kernel is twoscore to l times its original size. To come across popcorn pop in slow motion, select from the Pop Videos bill of fare, "Popcorn in Slow Motion"2.
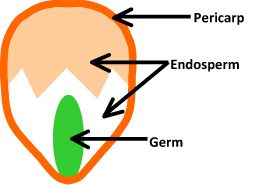 |
| Effigy 1 Corn kernel. |
People often wonder what is the platonic percentage of h2o in popcorn kernels for best popping. Popcorn is harvested in the fall when the kernels' wet content is between 16 and 20%. The kernels are then stored in bins where they are stale by forced air until reaching an optimum wet level of 14%. If the moisture content drops below that value, the size of the popped kernels is smaller and the number of kernels that pop decreases.
Research activity
Popcorn is a great real-globe case to use when discussing the kinetic molecular theory of gases, the phase change of water from a liquid to a gas, Gay-Lussac's gas law (pressure directly related to temperature), and the ideal gas police (PV = nRT). After covering the gas laws in grade, complement the lesson with an inquiry activity using several brands of popcorn. Accept pupil lab groups devise a lab procedure to decide the following and submit it for your approval:
- Decide the % of water by weight for 20 kernels of each make.
- Decide the internal pressure in atmospheres (atm) needed for each of the 20 kernels to pop. Hint: Utilize the platonic gas law, PV = nRT. Solve for P, which will be pressure in atm.
The other values in the equation will exist:
- n = Moles of water lost (g water lost after popping ÷ 18.0 thou/mol)
- R = Platonic gas police force abiding (0.0821 Fifty-atm/mol•K)
- T = Humid temperature of cooking oil in Kelvin (225° C + 273) = 498 Thousand
- V = Volume of 20 kernels (adamant by displacement of 5 mL h2o in a 10- or 25-mL graduated cylinder.)
Materials (for xxx students working in pairs)
- 15 Beakers (250 mL)
- xv Graduated Cylinders (ten or 25 mL)
- 15 Wire Gauzes
- 15 Ring Stands (with fe rings)
- 15 Bunsen Burners or Hot Plates
- xv Weighing Boats
Take the following materials available at a fundamental location:
Safety
Students should wear goggles and aprons or lab coats during the action and exercise due caution effectually Bunsen burners or hot plates. Inform students that cooking oil boils at a higher temperature than h2o (225° C) and accept them cover their beakers with aluminum foil to contain the popping corn and humid oil. Annotation: Remind students non to consume any of the popcorn produced in the lab.
Helpful hints
- Students should add together merely enough oil to embrace the bottom of their beaker earlier adding the 20 kernels of popcorn.
- Mass twenty kernels in a weighing gunkhole and decrease out the weighing boat's mass.
- Mass the beaker, oil, and 20 kernels before heating and without the foil covering.
- Mass the room temperature beaker, oil, and popcorn after heating and without the foil.
Sample data
| Mass of xx kernels + weighing boat | 4.26 yard |
| Mass of weighing boat | 2.xvi yard |
| Mass of popcorn | ii.x g |
| Book of popcorn (5.0 mL water displaced to 6.five mL) | 1.5 mL |
| Volume of popcorn in L | 0.0015 L |
| Mass of chalice, oil, and kernels | 105.55 1000 |
| Mass of chalice, oil, and popped popcorn at room temperature | 105.35 g |
| Mass of h2o lost | 0.twenty g |
| Moles of h2o lost = 0.20 g ÷ 18.0 g/mol | 0.011 mol |
Sample calculations
- % of water in kernels = water lost ÷ mass of kernels × 100
0.twenty yard ÷ two.10 grand × 100 = 9.5%
- Pressure inside of kernel prior to popping PV = nRT
P = nRT
Five
P =
(0.011 mol) (0.0821 L•atm/mol•K) (498 One thousand)0.0015 L
P = 3.0 × 10two atm
Conclusion/discussion of errors
Most literature cites popping pressure as 9.two atm (135 psi). If you choose to give your students that value, have them wait at their measurements and see where some weaknesses may lie. An obvious weakness is the number of popcorn kernels. The more kernels used, the more accurate the measurements for volume and loss of water will exist due to more significant digits. The temperature of the boiling oil is causeless to be 225° C, and the kernels are assumed to all pop at the same temperature.
Notes
- American Chemical Social club, "Popcorn: The snack with fifty-fifty higher antioxidants levels than fruits and vegetables"
- The Popcorn Lath, "Popcorn in Slow Motility"
What Makes Popcorn Pop Background Research,
Source: https://www.carolina.com/teacher-resources/Interactive/the-science-of-popcorn/tr23952.tr
Posted by: wayswee1997.blogspot.com


0 Response to "What Makes Popcorn Pop Background Research"
Post a Comment