Is Nitrogen A Noble Gas
| Noble gases | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
halogens ← → alkali metals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ↓Period | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | ii | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| five | 54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Radon (Rn) 86 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| seven | Oganesson (Og) 118 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Legend
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The noble gases (historically too the inert gases; sometimes referred to as aerogens [1]) make up a class of chemical elements with similar properties; under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The half-dozen naturally occurring noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).
Oganesson (Og) is a synthetically produced highly radioactive element, variously predicted to be another noble gas, or to intermission the trend and be reactive, due to relativistic effects. In part due to the extremely short 0.vii ms half-life of its only known isotope, its chemistry has non yet been investigated.
For the first six periods of the periodic table, the noble gases are exactly the members of group eighteen. Noble gases are typically highly unreactive except when nether particular extreme conditions. The inertness of noble gases makes them very suitable in applications where reactions are not wanted. For example, argon is used in incandescent lamps to forestall the hot tungsten filament from oxidizing; besides, helium is used in animate gas by abyssal divers to forbid oxygen, nitrogen and carbon dioxide toxicity.
The properties of the noble gases tin be well explained by modern theories of atomic structure: Their outer shell of valence electrons is considered to exist "total", giving them little trend to participate in chemical reactions, and it has been possible to prepare just a few hundred noble gas compounds. The melting and humid points for a given noble gas are close together, differing past less than 10 °C (18 °F); that is, they are liquids over but a small temperature range.
Neon, argon, krypton, and xenon are obtained from air in an air separation unit using the methods of liquefaction of gases and fractional distillation. Helium is sourced from natural gas fields that have high concentrations of helium in the natural gas, using cryogenic gas separation techniques, and radon is ordinarily isolated from the radioactive decay of dissolved radium, thorium, or uranium compounds. Noble gases have several of import applications in industries such equally lighting, welding, and infinite exploration. A helium-oxygen breathing gas is often used past deep-sea divers at depths of seawater over 55 thousand (180 ft). Subsequently the risks caused by the flammability of hydrogen became apparent in the Hindenburg disaster, information technology was replaced with helium in blimps and balloons.
History [edit]
Noble gas is translated from the German language noun Edelgas , first used in 1898 by Hugo Erdmann[two] to indicate their extremely low level of reactivity. The name makes an analogy to the term "noble metals", which also have low reactivity. The noble gases have also been referred to as inert gases, but this characterization is deprecated as many noble gas compounds are now known.[3] Rare gases is another term that was used,[four] but this is also inaccurate because argon forms a fairly considerable part (0.94% past volume, i.3% past mass) of the Globe's temper due to disuse of radioactive potassium-40.[5]
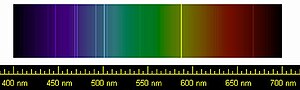
Helium was first detected in the Sun due to its characteristic spectral lines.
Pierre Janssen and Joseph Norman Lockyer had discovered a new element on eighteen August 1868 while looking at the chromosphere of the Dominicus, and named it helium after the Greek word for the Sun, ἥλιος ( hḗlios ).[half dozen] No chemical analysis was possible at the fourth dimension, but helium was later found to exist a element of group 0. Before them, in 1784, the English chemist and physicist Henry Cavendish had discovered that air contains a small proportion of a substance less reactive than nitrogen.[vii] A century afterward, in 1895, Lord Rayleigh discovered that samples of nitrogen from the air were of a different density than nitrogen resulting from chemical reactions. Along with Scottish scientist William Ramsay at University College, London, Lord Rayleigh theorized that the nitrogen extracted from air was mixed with another gas, leading to an experiment that successfully isolated a new element, argon, from the Greek word ἀργός ( argós , "idle" or "lazy").[seven] With this discovery, they realized an entire class of gases was missing from the periodic table. During his search for argon, Ramsay also managed to isolate helium for the first fourth dimension while heating cleveite, a mineral. In 1902, having accepted the show for the elements helium and argon, Dmitri Mendeleev included these noble gases equally group 0 in his system of the elements, which would later become the periodic table.[8]
Ramsay continued his search for these gases using the method of fractional distillation to divide liquid air into several components. In 1898, he discovered the elements krypton, neon, and xenon, and named them after the Greek words κρυπτός ( kryptós , "subconscious"), νέος ( néos , "new"), and ξένος ( ksénos , "stranger"), respectively. Radon was first identified in 1898 by Friedrich Ernst Dorn,[ix] and was named radium emanation, but was non considered a noble gas until 1904 when its characteristics were found to be similar to those of other noble gases.[10] Rayleigh and Ramsay received the 1904 Nobel Prizes in Physics and in Chemistry, respectively, for their discovery of the noble gases;[11] [12] in the words of J. E. Cederblom, so president of the Imperial Swedish Academy of Sciences, "the discovery of an entirely new group of elements, of which no single representative had been known with any certainty, is something utterly unique in the history of chemistry, being intrinsically an advance in science of peculiar significance".[12]
The discovery of the noble gases aided in the evolution of a full general understanding of atomic structure. In 1895, French chemist Henri Moissan attempted to form a reaction betwixt fluorine, the most electronegative element, and argon, one of the noble gases, but failed. Scientists were unable to prepare compounds of argon until the stop of the 20th century, but these attempts helped to develop new theories of diminutive structure. Learning from these experiments, Danish physicist Niels Bohr proposed in 1913 that the electrons in atoms are arranged in shells surrounding the nucleus, and that for all noble gases except helium the outermost crush always contains eight electrons.[10] In 1916, Gilbert Due north. Lewis formulated the octet dominion, which ended an octet of electrons in the outer shell was the most stable organisation for whatever cantlet; this organisation caused them to be unreactive with other elements since they did not require any more electrons to complete their outer shell.[xiii]
In 1962, Neil Bartlett discovered the showtime chemical chemical compound of a noble gas, xenon hexafluoroplatinate.[14] Compounds of other noble gases were discovered soon after: in 1962 for radon, radon difluoride (RnF
2 ),[15] which was identified past radiotracer techniques and in 1963 for krypton, krypton difluoride (KrF
ii ).[sixteen] The first stable compound of argon was reported in 2000 when argon fluorohydride (HArF) was formed at a temperature of 40 K (−233.2 °C; −387.vii °F).[17]
In October 2006, scientists from the Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory successfully created synthetically oganesson, the 7th element in group 18,[18] past bombarding californium with calcium.[19]
Physical and atomic properties [edit]
| Property[x] [20] | Helium | Neon | Argon | Krypton | Xenon | Radon | Oganesson |
|---|---|---|---|---|---|---|---|
| Density (g/dmthree) | 0.1786 | 0.9002 | 1.7818 | 3.708 | 5.851 | 9.97 | 7200 (predicted)[21] |
| Humid point (G) | iv.four | 27.3 | 87.4 | 121.5 | 166.half dozen | 211.5 | 450±x (predicted)[21] |
| Melting point (Yard) | –[22] | 24.7 | 83.6 | 115.8 | 161.seven | 202.ii | 325±15 (predicted)[21] |
| Enthalpy of vaporization (kJ/mol) | 0.08 | one.74 | 6.52 | 9.05 | 12.65 | 18.1 | – |
| Solubility in water at 20 °C (cm3/kg) | 8.61 | 10.5 | 33.half dozen | 59.4 | 108.one | 230 | – |
| Atomic number | 2 | x | 18 | 36 | 54 | 86 | 118 |
| Diminutive radius (calculated) (pm) | 31 | 38 | 71 | 88 | 108 | 120 | – |
| Ionization free energy (kJ/mol) | 2372 | 2080 | 1520 | 1351 | 1170 | 1037 | 839 (predicted)[23] |
| Electronegativity[24] | 4.16 | 4.79 | 3.24 | 2.97 | 2.58 | 2.lx | – |
The noble gases have weak interatomic force, and consequently have very low melting and boiling points. They are all monatomic gases under standard conditions, including the elements with larger atomic masses than many commonly solid elements.[x] Helium has several unique qualities when compared with other elements: its boiling signal at 1 atm is lower than those of any other known substance; it is the but element known to exhibit superfluidity; and, information technology is the only element that cannot be solidified by cooling at atmospheric force per unit area[25] (an outcome explained by quantum mechanics as its goose egg point free energy is too loftier to permit freezing)[26] – a pressure of 25 standard atmospheres (two,500 kPa; 370 psi) must be applied at a temperature of 0.95 K (−272.200 °C; −457.960 °F) to convert it to a solid[25] while a pressure of about 115 kbar is required at room temperature.[27] The noble gases upwardly to xenon take multiple stable isotopes. Radon has no stable isotopes; its longest-lived isotope, 222Rn, has a half-life of three.viii days and decays to form helium and polonium, which ultimately decays to lead.[10] Melting and boiling points increase going down the grouping.

This is a plot of ionization potential versus atomic number. The noble gases, which are labeled, take the largest ionization potential for each menses.
The noble gas atoms, like atoms in virtually groups, increase steadily in atomic radius from one period to the next due to the increasing number of electrons. The size of the cantlet is related to several properties. For example, the ionization potential decreases with an increasing radius because the valence electrons in the larger noble gases are further away from the nucleus and are therefore non held as tightly together by the atom. Noble gases take the largest ionization potential among the elements of each period, which reflects the stability of their electron configuration and is related to their relative lack of chemical reactivity.[20] Some of the heavier noble gases, however, accept ionization potentials small enough to be comparable to those of other elements and molecules. It was the insight that xenon has an ionization potential similar to that of the oxygen molecule that led Bartlett to attempt oxidizing xenon using platinum hexafluoride, an oxidizing agent known to exist potent enough to react with oxygen.[14] Noble gases cannot accept an electron to grade stable anions; that is, they have a negative electron affinity.[28]
The macroscopic physical properties of the noble gases are dominated by the weak van der Waals forces between the atoms. The attractive force increases with the size of the atom as a consequence of the increase in polarizability and the decrease in ionization potential. This results in systematic grouping trends: as 1 goes downwards group eighteen, the atomic radius, and with it the interatomic forces, increases, resulting in an increasing melting point, boiling point, enthalpy of vaporization, and solubility. The increase in density is due to the increase in atomic mass.[20]
The noble gases are nearly ideal gases under standard conditions, merely their deviations from the ideal gas constabulary provided important clues for the study of intermolecular interactions. The Lennard-Jones potential, often used to model intermolecular interactions, was deduced in 1924 by John Lennard-Jones from experimental data on argon before the development of quantum mechanics provided the tools for understanding intermolecular forces from get-go principles.[29] The theoretical analysis of these interactions became tractable because the noble gases are monatomic and the atoms spherical, which means that the interaction betwixt the atoms is contained of direction, or isotropic.
Chemical properties [edit]

Neon, like all noble gases, has a total valence shell. Noble gases take eight electrons in their outermost shell, except in the case of helium, which has two.
The noble gases are colorless, odorless, tasteless, and nonflammable under standard weather.[thirty] They were once labeled group 0 in the periodic table because it was believed they had a valence of zero, meaning their atoms cannot combine with those of other elements to form compounds. All the same, information technology was afterwards discovered some do indeed form compounds, causing this characterization to autumn into disuse.[10]
Electron configuration [edit]
Like other groups, the members of this family bear witness patterns in its electron configuration, especially the outermost shells resulting in trends in chemic behavior:
| Z | Chemical element | No. of electrons/crush |
|---|---|---|
| 2 | helium | ii |
| x | neon | ii, viii |
| eighteen | argon | 2, 8, 8 |
| 36 | krypton | 2, viii, 18, 8 |
| 54 | xenon | 2, 8, 18, eighteen, 8 |
| 86 | radon | ii, 8, eighteen, 32, xviii, eight |
| 118 | oganesson | ii, 8, 18, 32, 32, 18, eight (predicted) |
The noble gases accept full valence electron shells. Valence electrons are the outermost electrons of an atom and are normally the only electrons that participate in chemical bonding. Atoms with full valence electron shells are extremely stable and therefore do non tend to form chemic bonds and take little tendency to proceeds or lose electrons.[31] Even so, heavier noble gases such as radon are held less firmly together by electromagnetic force than lighter noble gases such as helium, making it easier to remove outer electrons from heavy noble gases.
Equally a result of a full shell, the noble gases can be used in conjunction with the electron configuration notation to course the noble gas notation. To do this, the nearest noble gas that precedes the element in question is written first, and then the electron configuration is connected from that betoken forrard. For example, the electron notation of phosphorus is 1s2 2s2 2p6 3sii 3p3 , while the noble gas notation is [Ne] 3sii 3piii . This more than compact notation makes information technology easier to identify elements, and is shorter than writing out the full notation of atomic orbitals.[32]
The noble gases cantankerous the boundary between blocks—helium is an s-element whereas the rest of members are p-elements—which is unusual amongst the IUPAC groups. Almost, if non all[33] other IUPAC groups contain elements from one block each.
Compounds [edit]
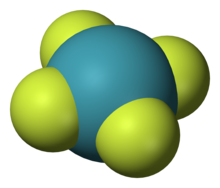
Structure of XeF
4 , one of the beginning noble gas compounds to be discovered
The noble gases show extremely depression chemical reactivity; consequently, only a few hundred element of group 0 compounds have been formed. Neutral compounds in which helium and neon are involved in chemical bonds accept not been formed (although some helium-containing ions exist and there is some theoretical evidence for a few neutral helium-containing ones), while xenon, krypton, and argon have shown only pocket-sized reactivity.[34] The reactivity follows the order Ne < He < Ar < Kr < Xe < Rn ≪ Og.
In 1933, Linus Pauling predicted that the heavier noble gases could form compounds with fluorine and oxygen. He predicted the existence of krypton hexafluoride (KrF
six ) and xenon hexafluoride (XeF
half dozen ), speculated that XeF
8 might exist as an unstable compound, and suggested that xenic acid could course perxenate salts.[35] [36] These predictions were shown to exist generally accurate, except that XeF
viii is at present thought to be both thermodynamically and kinetically unstable.[37]
Xenon compounds are the most numerous of the noble gas compounds that have been formed.[38] Nigh of them take the xenon atom in the oxidation state of +2, +iv, +6, or +8 bonded to highly electronegative atoms such as fluorine or oxygen, as in xenon difluoride (XeF
ii ), xenon tetrafluoride (XeF
4 ), xenon hexafluoride (XeF
6 ), xenon tetroxide (XeO
4 ), and sodium perxenate (Na
4 XeO
vi ). Xenon reacts with fluorine to form numerous xenon fluorides according to the following equations:
-
- Xe + F2 → XeF2
- Xe + 2Fii → XeF4
- Xe + 3Fii → XeF6
Some of these compounds take found use in chemic synthesis as oxidizing agents; XeF
2 , in particular, is commercially available and tin can be used as a fluorinating amanuensis.[39] As of 2007, about five hundred compounds of xenon bonded to other elements take been identified, including organoxenon compounds (containing xenon bonded to carbon), and xenon bonded to nitrogen, chlorine, golden, mercury, and xenon itself.[34] [xl] Compounds of xenon bound to boron, hydrogen, bromine, iodine, beryllium, sulphur, titanium, copper, and silver have also been observed but but at low temperatures in noble gas matrices, or in supersonic noble gas jets.[34]
Radon is more than reactive than xenon, and forms chemical bonds more easily than xenon does. However, due to the loftier radioactivity and short half-life of radon isotopes, only a few fluorides and oxides of radon have been formed in practice.[41] Radon goes further towards metallic beliefs than xenon; the difluoride RnF2 is highly ionic, and cationic Rn2+ is formed in halogen fluoride solutions. For this reason, kinetic hindrance makes it difficult to oxidize radon beyond the +ii state. Only tracer experiments appear to have succeeded in doing and then, probably forming RnF4, RnF6, and RnOthree.[42] [43] [44]
Krypton is less reactive than xenon, but several compounds have been reported with krypton in the oxidation land of +2.[34] Krypton difluoride is the most notable and hands characterized. Under extreme conditions, krypton reacts with fluorine to grade KrFtwo according to the following equation:
-
- Kr + F2 → KrF2
Compounds in which krypton forms a single bail to nitrogen and oxygen take also been characterized,[45] but are only stable below −sixty °C (−76 °F) and −ninety °C (−130 °F) respectively.[34]
Krypton atoms chemically leap to other nonmetals (hydrogen, chlorine, carbon) as well as some late transition metals (copper, silver, gilt) have besides been observed, merely merely either at low temperatures in noble gas matrices, or in supersonic noble gas jets.[34] Like conditions were used to obtain the first few compounds of argon in 2000, such every bit argon fluorohydride (HArF), and some bound to the tardily transition metals copper, silver, and gold.[34] As of 2007, no stable neutral molecules involving covalently bound helium or neon are known.[34]
Extrapolation from periodic trends predict that oganesson should be the nearly reactive of the noble gases; more sophisticated theoretical treatments indicate greater reactivity than such extrapolations propose, to the signal where the applicability of the descriptor "noble gas" has been questioned.[46] Oganesson is expected to exist rather like silicon or can in group 14:[47] a reactive chemical element with a common +4 and a less common +2 state,[48] [49] which at room temperature and pressure is not a gas but rather a solid semiconductor. Empirical / experimental testing will be required to validate these predictions.[21] [50] (On the other paw, flerovium, despite being in group 14, is predicted to exist unusually volatile, which suggests noble gas-like properties.[51] [52]).
The noble gases—including helium—can grade stable molecular ions in the gas phase. The simplest is the helium hydride molecular ion, HeH+, discovered in 1925.[53] Because it is equanimous of the two most abundant elements in the universe, hydrogen and helium, information technology is believed to occur naturally in the interstellar medium, although it has not been detected notwithstanding.[54] In addition to these ions, there are many known neutral excimers of the noble gases. These are compounds such as ArF and KrF that are stable only when in an excited electronic land; some of them detect application in excimer lasers.
In addition to the compounds where a noble gas cantlet is involved in a covalent bond, noble gases also form non-covalent compounds. The clathrates, outset described in 1949,[55] consist of a element of group 0 cantlet trapped within cavities of crystal lattices of certain organic and inorganic substances. The essential condition for their formation is that the invitee (element of group 0) atoms must exist of advisable size to fit in the cavities of the host crystal lattice. For instance, argon, krypton, and xenon form clathrates with hydroquinone, merely helium and neon do not considering they are too small or insufficiently polarizable to be retained.[56] Neon, argon, krypton, and xenon also form clathrate hydrates, where the element of group 0 is trapped in ice.[57]
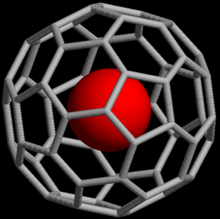
An endohedral fullerene compound containing a noble gas atom
Noble gases can form endohedral fullerene compounds, in which the noble gas atom is trapped inside a fullerene molecule. In 1993, it was discovered that when C
60 , a spherical molecule consisting of lx carbon atoms, is exposed to noble gases at high pressure, complexes such as He@C
threescore can be formed (the @ notation indicates He is contained within C
lx only not covalently bound to it).[58] Every bit of 2008, endohedral complexes with helium, neon, argon, krypton, and xenon have been created.[59] These compounds take found use in the report of the construction and reactivity of fullerenes by means of the nuclear magnetic resonance of the element of group 0 atom.[60]

Bonding in XeF
2 according to the 3-centre-4-electron bail model
Noble gas compounds such as xenon difluoride (XeF
two ) are considered to be hypervalent because they violate the octet rule. Bonding in such compounds can be explained using a three-center iv-electron bond model.[61] [62] This model, first proposed in 1951, considers bonding of iii collinear atoms. For case, bonding in XeF
2 is described by a set of three molecular orbitals (MOs) derived from p-orbitals on each atom. Bonding results from the combination of a filled p-orbital from Xe with 1 one-half-filled p-orbital from each F atom, resulting in a filled bonding orbital, a filled non-bonding orbital, and an empty antibonding orbital. The highest occupied molecular orbital is localized on the ii final atoms. This represents a localization of charge that is facilitated by the loftier electronegativity of fluorine.[63]
The chemistry of the heavier noble gases, krypton and xenon, are well established. The chemistry of the lighter ones, argon and helium, is nonetheless at an early stage, while a neon chemical compound is yet to exist identified.
Occurrence and production [edit]
The abundances of the noble gases in the universe decrease equally their diminutive numbers increment. Helium is the most common element in the universe afterwards hydrogen, with a mass fraction of about 24%. Nearly of the helium in the universe was formed during Large Bang nucleosynthesis, but the amount of helium is steadily increasing due to the fusion of hydrogen in stellar nucleosynthesis (and, to a very slight degree, the alpha decay of heavy elements).[64] [65] Abundances on Earth follow different trends; for case, helium is but the third virtually abundant noble gas in the atmosphere. The reason is that in that location is no primordial helium in the temper; due to the modest mass of the atom, helium cannot be retained by the World's gravitational field.[66] Helium on Earth comes from the blastoff decay of heavy elements such as uranium and thorium institute in the Earth's crust, and tends to accrue in natural gas deposits.[66] The abundance of argon, on the other hand, is increased equally a upshot of the beta decay of potassium-40, as well found in the Earth'southward crust, to form argon-40, which is the near abundant isotope of argon on Globe despite being relatively rare in the Solar System. This process is the basis for the potassium-argon dating method.[67] Xenon has an unexpectedly low abundance in the atmosphere, in what has been called the missing xenon problem; 1 theory is that the missing xenon may be trapped in minerals inside the Earth'south crust.[68] After the discovery of xenon dioxide, research showed that Xe tin can substitute for Si in quartz.[69] Radon is formed in the lithosphere past the alpha decay of radium. It can seep into buildings through cracks in their foundation and accumulate in areas that are not well ventilated. Due to its high radioactivity, radon presents a meaning wellness hazard; it is implicated in an estimated 21,000 lung cancer deaths per twelvemonth in the United states of america alone.[70] Oganesson does non occur in nature and is instead created manually by scientists.
| Abundance | Helium | Neon | Argon | Krypton | Xenon | Radon |
|---|---|---|---|---|---|---|
| Solar System (for each atom of silicon)[71] | 2343 | 2.148 | 0.1025 | 5.515 × 10−5 | 5.391 × 10−6 | – |
| Earth'south atmosphere (volume fraction in ppm)[72] | 5.20 | 18.twenty | 9340.00 | 1.10 | 0.09 | (0.06–eighteen) × 10−19 [73] |
| Igneous rock (mass fraction in ppm)[20] | 3 × 10−3 | 7 × ten−5 | four × 10−two | – | – | one.7 × 10−10 |
| Gas | 2004 price (USD/m3)[74] |
|---|---|
| Helium (industrial grade) | 4.xx–4.90 |
| Helium (laboratory grade) | 22.30–44.90 |
| Argon | two.70–8.50 |
| Neon | 60–120 |
| Krypton | 400–500 |
| Xenon | 4000–5000 |
For large-calibration use, helium is extracted past fractional distillation from natural gas, which can contain up to 7% helium.[75]
Neon, argon, krypton, and xenon are obtained from air using the methods of liquefaction of gases, to convert elements to a liquid state, and fractional distillation, to split up mixtures into component parts. Helium is typically produced past separating it from natural gas, and radon is isolated from the radioactivity of radium compounds.[10] The prices of the noble gases are influenced by their natural abundance, with argon beingness the cheapest and xenon the most expensive. Every bit an example, the adjacent table lists the 2004 prices in the U.s. for laboratory quantities of each gas.
Applications [edit]

Liquid helium is used to absurd superconducting magnets in modern MRI scanners
Noble gases have very depression humid and melting points, which makes them useful as cryogenic refrigerants.[76] In detail, liquid helium, which boils at 4.2 Chiliad (−268.95 °C; −452.11 °F), is used for superconducting magnets, such every bit those needed in nuclear magnetic resonance imaging and nuclear magnetic resonance.[77] Liquid neon, although it does not reach temperatures every bit low as liquid helium, also finds use in cryogenics because it has over 40 times more refrigerating capacity than liquid helium and over three times more than than liquid hydrogen.[73]
Helium is used equally a component of breathing gases to replace nitrogen, due its low solubility in fluids, especially in lipids. Gases are captivated by the blood and body tissues when nether force per unit area similar in scuba diving, which causes an anesthetic issue known as nitrogen narcosis.[78] Due to its reduced solubility, piffling helium is taken into jail cell membranes, and when helium is used to replace part of the breathing mixtures, such as in trimix or heliox, a subtract in the narcotic outcome of the gas at depth is obtained.[79] Helium's reduced solubility offers further advantages for the condition known as decompression sickness, or the bends.[10] [eighty] The reduced amount of dissolved gas in the trunk means that fewer gas bubbles form during the decrease in pressure of the ascension. Another noble gas, argon, is considered the best pick for use as a drysuit aggrandizement gas for scuba diving.[81] Helium is besides used as filling gas in nuclear fuel rods for nuclear reactors.[82]

Since the Hindenburg disaster in 1937,[83] helium has replaced hydrogen as a lifting gas in blimps and balloons due to its lightness and incombustibility, despite an 8.6%[84] decrease in buoyancy.[10]
In many applications, the noble gases are used to provide an inert atmosphere. Argon is used in the synthesis of air-sensitive compounds that are sensitive to nitrogen. Solid argon is also used for the study of very unstable compounds, such as reactive intermediates, past trapping them in an inert matrix at very low temperatures.[85] Helium is used equally the carrier medium in gas chromatography, every bit a filler gas for thermometers, and in devices for measuring radiation, such as the Geiger counter and the bubble sleeping accommodation.[74] Helium and argon are both ordinarily used to shield welding arcs and the surrounding base metal from the atmosphere during welding and cutting, as well every bit in other metallurgical processes and in the production of silicon for the semiconductor industry.[73]

Noble gases are usually used in lighting because of their lack of chemical reactivity. Argon, mixed with nitrogen, is used as a filler gas for incandescent light bulbs.[73] Krypton is used in high-operation light bulbs, which have higher color temperatures and greater efficiency, considering it reduces the charge per unit of evaporation of the filament more than than argon; halogen lamps, in particular, use krypton mixed with small-scale amounts of compounds of iodine or bromine.[73] The noble gases glow in distinctive colors when used within gas-discharge lamps, such as "neon lights". These lights are called after neon but frequently contain other gases and phosphors, which add various hues to the orangish-reddish colour of neon. Xenon is commonly used in xenon arc lamps, which, due to their virtually continuous spectrum that resembles daylight, find application in film projectors and as automobile headlamps.[73]
The noble gases are used in excimer lasers, which are based on short-lived electronically excited molecules known as excimers. The excimers used for lasers may exist element of group 0 dimers such as Ar2, Krii or Xe2, or more unremarkably, the element of group 0 is combined with a element of group vii in excimers such as ArF, KrF, XeF, or XeCl. These lasers produce ultraviolet lite, which, due to its short wavelength (193 nm for ArF and 248 nm for KrF), allows for high-precision imaging. Excimer lasers take many industrial, medical, and scientific applications. They are used for microlithography and microfabrication, which are essential for integrated circuit manufacture, and for laser surgery, including laser angioplasty and centre surgery.[86]
Some noble gases accept directly application in medicine. Helium is sometimes used to improve the ease of breathing of asthma sufferers.[73] Xenon is used as an coldhearted considering of its high solubility in lipids, which makes it more than stiff than the usual nitrous oxide, and considering it is readily eliminated from the body, resulting in faster recovery.[87] Xenon finds application in medical imaging of the lungs through hyperpolarized MRI.[88] Radon, which is highly radioactive and is only available in minute amounts, is used in radiotherapy.[10]
Noble gases, particularly xenon, are predominantly used in ion engines due to their inertness. Since ion engines are non driven by chemical reactions, chemically inert fuels are desired to prevent unwanted reaction between the fuel and anything else on the engine.
Oganesson is also unstable to work with and has no known application other than research.
Discharge color [edit]
 |  |  |  |  |
 |  |  |  |  |
 |  |  |  |  |
| | | | | |
| Helium | Neon | Argon | Krypton | Xenon |
The color of gas discharge emission depends on several factors, including the post-obit:[89]
- discharge parameters (local value of electric current density and electrical field, temperature, etc. – note the color variation along the discharge in the top row);
- gas purity (even small fraction of sure gases can affect color);
- cloth of the belch tube envelope – note suppression of the UV and blue components in the bottom-row tubes fabricated of thick household drinking glass.
Run across also [edit]
- Noble gas (data folio), for extended tables of physical backdrop.
- Noble metal, for metals that are resistant to corrosion or oxidation.
- Inert gas, for any gas that is non reactive under normal circumstances.
- Industrial gas
- Neutronium
- Octet rule
Notes [edit]
- ^ Bauzá, Antonio; Frontera, Antonio (2015). "Aerogen Bonding Interaction: A New Supramolecular Force?". Angewandte Chemie International Edition. 54 (25): 7340–3. doi:10.1002/anie.201502571. PMID 25950423.
- ^ Renouf, Edward (1901). "Noble gases". Science. 13 (320): 268–270. Bibcode:1901Sci....13..268R. doi:ten.1126/science.13.320.268. S2CID 34534533.
- ^ Ozima 2002, p. xxx
- ^ Ozima 2002, p. iv
- ^ "argon". Encyclopædia Britannica. 2008.
- ^ Oxford English Dictionary (1989), s.five. "helium". Retrieved 16 December 2006, from Oxford English Dictionary Online. Also, from quotation at that place: Thomson, W. (1872). Rep. Brit. Assoc. xcix: "Frankland and Lockyer find the yellow prominences to give a very decided brilliant line not far from D, only hitherto not identified with whatsoever terrestrial flame. It seems to indicate a new substance, which they propose to call Helium."
- ^ a b Ozima 2002, p. ane
- ^ Mendeleev 1903, p. 497
- ^ Partington, J. R. (1957). "Discovery of Radon". Nature. 179 (4566): 912. Bibcode:1957Natur.179..912P. doi:10.1038/179912a0. S2CID 4251991.
- ^ a b c d due east f m h i j "Noble gas". Encyclopædia Britannica. 2008.
- ^ Cederblom, J. Due east. (1904). "The Nobel Prize in Physics 1904 Presentation Spoken communication".
- ^ a b Cederblom, J. Due east. (1904). "The Nobel Prize in Chemistry 1904 Presentation Speech".
- ^ Gillespie, R. J.; Robinson, East. A. (2007). "Gilbert North. Lewis and the chemical bond: the electron pair and the octet dominion from 1916 to the present twenty-four hours". J Comput Chem. 28 (1): 87–97. doi:10.1002/jcc.20545. PMID 17109437.
- ^ a b Bartlett, N. (1962). "Xenon hexafluoroplatinate Xe+[PtF6]−". Proceedings of the Chemical Society (6): 218. doi:x.1039/PS9620000197.
- ^ Fields, Paul R.; Stein, Lawrence; Zirin, Moshe H. (1962). "Radon Fluoride". Journal of the American Chemical Social club. 84 (21): 4164–4165. doi:10.1021/ja00880a048.
- ^ Grosse, A. V.; Kirschenbaum, A. D.; Streng, A. M.; Streng, 50. V. (1963). "Krypton Tetrafluoride: Preparation and Some Backdrop". Scientific discipline. 139 (3559): 1047–1048. Bibcode:1963Sci...139.1047G. doi:10.1126/science.139.3559.1047. PMID 17812982.
- ^
- ^ Hairdresser, Robert C.; Karol, Paul J.; Nakahara, Hiromichi; Vardaci, Emanuele & Vogt, Erich W. (2011). "Discovery of the elements with atomic numbers greater than or equal to 113 (IUPAC Technical Report)*" (PDF). Pure Appl. Chem. IUPAC. 83 (7). doi:ten.1515/ci.2011.33.5.25b. Retrieved 30 May 2014.
- ^ Oganessian, Yu. Ts.; Utyonkov, 5.; Lobanov, Yu.; Abdullin, F.; Polyakov, A.; et al. (2006). "Synthesis of the isotopes of elements 118 and 116 in the 249
Cf
and 245
Cm
+ 48
Ca
fusion reactions". Physical Review C. 74 (4): 44602. Bibcode:2006PhRvC..74d4602O. doi:10.1103/PhysRevC.74.044602. - ^ a b c d Greenwood 1997, p. 891
- ^ a b c d Smits, Odile; Mewes, Jan-Michael; Jerabek, Paul; Schwerdtfeger, Peter (2020). "Oganesson: A Noble Gas Element That Is Neither Noble Nor a Gas". Angew. Chem. Int. Ed. 59 (52): 23636–23640. doi:10.1002/anie.202011976. PMC7814676. PMID 32959952.
- ^ Liquid helium will only solidify if exposed to pressures well to a higher place atmospheric force per unit area, an effect explainable with quantum mechanics
- ^ Wintertime, Marker (2020). "Organesson: Properties of Free Atoms". WebElements: THE periodic table on the World wide web . Retrieved 30 December 2020.
- ^ Allen, Leland C. (1989). "Electronegativity is the average one-electron energy of the valence-shell electrons in ground-land free atoms". Journal of the American Chemical Society. 111 (25): 9003–9014. doi:ten.1021/ja00207a003.
- ^ a b Wilks, John (1967). "Introduction". The Properties of Liquid and Solid Helium. Oxford: Clarendon Press. ISBN978-0-19-851245-five.
- ^ "John Beamish'southward Enquiry on Solid Helium". Department of Physics, University of Alberta. 2008. Archived from the original on 31 May 2008.
- ^ Pinceaux, J.-P.; Maury, J.-P.; Besson, J.-M. (1979). "Solidification of helium, at room temperature under high pressure level" (PDF). Periodical de Physique Lettres. twoscore (13): 307–308. doi:x.1051/jphyslet:019790040013030700. S2CID 40164915.
- ^ Wheeler, John C. (1997). "Electron Affinities of the Alkali metal World Metals and the Sign Convention for Electron Affinity". Journal of Chemic Instruction. 74 (1): 123–127. Bibcode:1997JChEd..74..123W. doi:ten.1021/ed074p123. ; Kalcher, Josef; Sax, Alexander F. (1994). "Gas Stage Stabilities of Small Anions: Theory and Experiment in Cooperation". Chemical Reviews. 94 (8): 2291–2318. doi:10.1021/cr00032a004.
- ^ Mott, N. F. (1955). "John Edward Lennard-Jones. 1894–1954". Biographical Memoirs of Fellows of the Purple Society. 1: 175–184. doi:ten.1098/rsbm.1955.0013.
- ^ Wiley-VCH (2003). Ullmann's Encyclopedia of Industrial Chemistry – Volume 23. John Wiley & Sons. p. 217.
- ^ Ozima 2002, p. 35
- ^ CliffsNotes 2007, p. 15
- ^ IUPAC cannot decide on exact composition of group 3 which include elements from the f-cake in some proposals. Their inclusion would make group three the second cross-block group.
- ^ a b c d e f g h Grochala, Wojciech (2007). "Atypical compounds of gases, which take been chosen noble" (PDF). Chemical Club Reviews. 36 (10): 1632–1655. doi:ten.1039/b702109g. PMID 17721587.
- ^ Pauling, Linus (1933). "The Formulas of Antimonic Acid and the Antimonates". Journal of the American Chemic Society. 55 (5): 1895–1900. doi:x.1021/ja01332a016.
- ^ Holloway 1968
- ^ Seppelt, Konrad (1979). "Recent developments in the Chemistry of Some Electronegative Elements". Accounts of Chemical Research. 12 (vi): 211–216. doi:10.1021/ar50138a004.
- ^ Moody, Chiliad. J. (1974). "A Decade of Xenon Chemistry". Periodical of Chemical Educational activity. 51 (ten): 628–630. Bibcode:1974JChEd..51..628M. doi:ten.1021/ed051p628. Retrieved 16 Oct 2007.
- ^ Zupan, Marko; Iskra, Jernej; Stavber, Stojan (1998). "Fluorination with XeF2. 44. Upshot of Geometry and Heteroatom on the Regioselectivity of Fluorine Introduction into an Aromatic Ring". J. Org. Chem. 63 (3): 878–880. doi:10.1021/jo971496e. PMID 11672087.
- ^ Harding 2002, pp. xc–99
- ^ .Avrorin, V. V.; Krasikova, R. North.; Nefedov, V. D.; Toropova, M. A. (1982). "The Chemistry of Radon". Russian Chemical Reviews. 51 (1): 12–20. Bibcode:1982RuCRv..51...12A. doi:x.1070/RC1982v051n01ABEH002787.
- ^ Stein, Lawrence (1983). "The Chemical science of Radon". Radiochimica Acta. 32 (ane–3): 163–171. doi:x.1524/ract.1983.32.13.163. S2CID 100225806.
- ^ Liebman, Joel F. (1975). "Conceptual Problems in Noble Gas and Fluorine Chemistry, II: The Nonexistence of Radon Tetrafluoride". Inorg. Nucl. Chem. Lett. eleven (10): 683–685. doi:ten.1016/0020-1650(75)80185-1.
- ^ Seppelt, Konrad (2015). "Molecular Hexafluorides". Chemical Reviews. 115 (two): 1296–1306. doi:10.1021/cr5001783. PMID 25418862.
- ^ Lehmann, J (2002). "The chemistry of krypton". Coordination Chemistry Reviews. 233–234: one–39. doi:x.1016/S0010-8545(02)00202-iii.
- ^ Roth, Klaus (2017). "Ist das Element 118 ein Edelgas?" [Is Element 118 a Noble Gas?]. Chemie in unserer Zeit (in German). 51 (6): 418–426. doi:10.1002/ciuz.201700838.
Translated into English by W. Due east. Russey and published in three parts in ChemViews Magazine:
Roth, Klaus (3 Apr 2018). "New Kids on the Table: Is Element 118 a Noble Gas? – Part one". ChemViews Magazine. doi:10.1002/chemv.201800029.
Roth, Klaus (one May 2018). "New Kids on the Table: Is Element 118 a Element of group 0? – Function 2". ChemViews Magazine. doi:ten.1002/chemv.201800033.
Roth, Klaus (v June 2018). "New Kids on the Tabular array: Is Element 118 a Noble Gas? – Role iii". ChemViews Mag. doi:10.1002/chemv.201800046. - ^ Kulsha, A. 5. "Есть ли граница у таблицы Менделеева?" [Is in that location a boundary to the Mendeleev tabular array?] (PDF). www.primefan.ru (in Russian). Retrieved 8 September 2018.
- ^ Fricke, Burkhard (1975). Superheavy elements: a prediction of their chemical and concrete properties. Contempo Impact of Physics on Inorganic Chemistry. Structure and Bonding. Vol. 21. pp. 89–144. doi:10.1007/BFb0116498. ISBN978-iii-540-07109-nine . Retrieved iv October 2013.
- ^ Russian periodic tabular array poster by A. V. Kulsha and T. A. Kolevich
- ^ Mewes, January-Michael; Smits, Odile Rosette; Jerabek, Paul; Schwerdtfeger, Peter (25 July 2019). "Oganesson is a Semiconductor: On the Relativistic Band‐Gap Narrowing in the Heaviest Noble‐Gas Solids". Angewandte Chemie. 58 (40): 14260–14264. doi:10.1002/anie.201908327. PMC6790653. PMID 31343819.
- ^ Kratz, J. Five. (1 August 2012). "The bear on of the properties of the heaviest elements on the chemical and physical sciences". Radiochimica Acta. 100 (8–9): 569–578. doi:10.1524/ract.2012.1963. ISSN 2193-3405. S2CID 97915854.
- ^ "Indication for a volatile chemical element 114" (PDF). doc.rero.ch.
{{cite web}}: CS1 maint: url-status (link) - ^ Hogness, T. R.; Lunn, E. One thousand. (1925). "The Ionization of Hydrogen by Electron Touch on as Interpreted by Positive Ray Assay". Concrete Review. 26 (1): 44–55. Bibcode:1925PhRv...26...44H. doi:10.1103/PhysRev.26.44.
- ^ Fernandez, J.; Martin, F. (2007). "Photoionization of the HeH2 + molecular ion". J. Phys. B: At. Mol. Opt. Phys. 40 (12): 2471–2480. Bibcode:2007JPhB...twoscore.2471F. doi:ten.1088/0953-4075/twoscore/12/020.
- ^ Powell, H. 1000. & Guter, M. (1949). "An Inert Gas Compound". Nature. 164 (4162): 240–241. Bibcode:1949Natur.164..240P. doi:10.1038/164240b0. PMID 18135950. S2CID 4134617.
- ^ Greenwood 1997, p. 893
- ^ Dyadin, Yuri A.; et al. (1999). "Clathrate hydrates of hydrogen and neon". Mendeleev Communications. 9 (v): 209–210. doi:10.1070/MC1999v009n05ABEH001104.
- ^ Saunders, M.; Jiménez-Vázquez, H. A.; Cantankerous, R. J.; Poreda, R. J. (1993). "Stable compounds of helium and neon. He@C60 and Ne@C60". Science. 259 (5100): 1428–1430. Bibcode:1993Sci...259.1428S. doi:10.1126/science.259.5100.1428. PMID 17801275. S2CID 41794612.
- ^ Saunders, Martin; Jimenez-Vazquez, Hugo A.; Cross, R. James; Mroczkowski, Stanley; Gross, Michael 50.; Giblin, Daryl Due east.; Poreda, Robert J. (1994). "Incorporation of helium, neon, argon, krypton, and xenon into fullerenes using high pressure". J. Am. Chem. Soc. 116 (5): 2193–2194. doi:x.1021/ja00084a089.
- ^ Frunzi, Michael; Cross, R. Jame; Saunders, Martin (2007). "Effect of Xenon on Fullerene Reactions". Journal of the American Chemic Society. 129 (43): 13343–6. doi:10.1021/ja075568n. PMID 17924634.
- ^ Greenwood 1997, p. 897
- ^ Weinhold 2005, pp. 275–306
- ^ Pimentel, M. C. (1951). "The Bonding of Trihalide and Bifluoride Ions by the Molecular Orbital Method". The Periodical of Chemical Physics. 19 (4): 446–448. Bibcode:1951JChPh..19..446P. doi:ten.1063/1.1748245.
- ^ Weiss, Achim. "Elements of the by: Large Bang Nucleosynthesis and observation". Max Planck Institute for Gravitational Physics. Archived from the original on 8 February 2007. Retrieved 23 June 2008.
- ^ Coc, A.; et al. (2004). "Updated Big Bang Nucleosynthesis confronted to WMAP observations and to the Abundance of Light Elements". Astrophysical Journal. 600 (ii): 544–552. arXiv:astro-ph/0309480. Bibcode:2004ApJ...600..544C. doi:x.1086/380121. S2CID 16276658.
- ^ a b Morrison, P.; Pino, J. (1955). "Radiogenic Origin of the Helium Isotopes in Rock". Annals of the New York Academy of Sciences. 62 (three): 71–92. Bibcode:1955NYASA..62...71M. doi:10.1111/j.1749-6632.1955.tb35366.x. S2CID 85015694.
- ^ Scherer, Alexandra (16 January 2007). "twoscoreAr/39Ar dating and errors". Technische Universität Bergakademie Freiberg. Archived from the original on 14 October 2007. Retrieved 26 June 2008.
- ^ Sanloup, Chrystèle; Schmidt, Burkhard C.; et al. (2005). "Retention of Xenon in Quartz and Earth's Missing Xenon". Science. 310 (5751): 1174–1177. Bibcode:2005Sci...310.1174S. doi:10.1126/science.1119070. PMID 16293758. S2CID 31226092.
- ^ Tyler Irving (May 2011). "Xenon Dioxide May Solve I of World'due south Mysteries". 50'Actualité chimique canadienne (Canadian Chemical News). Archived from the original on 9 February 2013. Retrieved 18 May 2012.
- ^ "A Citizen'southward Guide to Radon". U.Southward. Environmental Protection Bureau. 26 Nov 2007. Retrieved 26 June 2008.
- ^ Lodders, Katharina (ten July 2003). "Solar System Abundances and Condensation Temperatures of the Elements" (PDF). The Astrophysical Periodical. The American Astronomical Society. 591 (ii): 1220–1247. Bibcode:2003ApJ...591.1220L. doi:10.1086/375492. Archived from the original (PDF) on seven November 2015. Retrieved ane September 2015.
- ^ "The Atmosphere". National Weather Service. Retrieved i June 2008.
- ^ a b c d e f g Häussinger, Peter; Glatthaar, Reinhard; Rhode, Wilhelm; Kick, Helmut; Benkmann, Christian; Weber, Josef; Wunschel, Hans-Jörg; Stenke, Viktor; Leicht, Edith; Stenger, Hermann (2002). "Noble gases". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a17_485. ISBN3-527-30673-0.
- ^ a b Hwang, Shuen-Chen; Lein, Robert D.; Morgan, Daniel A. (2005). "Noble Gases". Kirk Othmer Encyclopedia of Chemical Engineering science. Wiley. pp. 343–383. doi:10.1002/0471238961.0701190508230114.a01.
- ^ Winter, Mark (2008). "Helium: the essentials". University of Sheffield. Retrieved 14 July 2008.
- ^ "Neon". Encarta. 2008.
- ^ Zhang, C. J.; Zhou, 10. T.; Yang, L. (1992). "Demountable coaxial gas-cooled current leads for MRI superconducting magnets". IEEE Transactions on Magnetics. IEEE. 28 (i): 957–959. Bibcode:1992ITM....28..957Z. doi:10.1109/twenty.120038.
- ^ Fowler, B.; Ackles, K. N.; Porlier, Chiliad. (1985). "Furnishings of inert gas narcosis on behavior—a disquisitional review". Undersea Biomed. Res. 12 (4): 369–402. ISSN 0093-5387. OCLC 2068005. PMID 4082343. Archived from the original on 25 December 2010. Retrieved 8 April 2008.
- ^ Bennett 1998, p. 176
- ^ Vann, R. D., ed. (1989). "The Physiological Footing of Decompression". 38th Undersea and Hyperbaric Medical Club Workshop. 75(Phys)vi-1-89: 437. Retrieved 31 May 2008.
- ^ Maiken, Eric (1 Baronial 2004). "Why Argon?". Decompression. Retrieved 26 June 2008.
- ^ Horhoianu, K.; Ionescu, D. V.; Olteanu, G. (1999). "Thermal behaviour of CANDU blazon fuel rods during steady state and transient operating conditions". Annals of Nuclear Energy. 26 (16): 1437–1445. doi:10.1016/S0306-4549(99)00022-5.
- ^ "Disaster Ascribed to Gas by Experts". The New York Times. 7 May 1937. p. 1.
- ^ Freudenrich, Craig (2008). "How Blimps Work". HowStuffWorks. Retrieved 3 July 2008.
- ^ Dunkin, I. R. (1980). "The matrix isolation technique and its application to organic chemistry". Chem. Soc. Rev. ix: 1–23. doi:10.1039/CS9800900001.
- ^ Basting, Dirk; Marowsky, Gerd (2005). Excimer Laser Engineering. Springer. ISBNthree-540-20056-8.
- ^ Sanders, Robert D.; Ma, Daqing; Maze, Mervyn (2005). "Xenon: elemental anaesthesia in clinical practice". British Medical Bulletin. 71 (1): 115–135. doi:10.1093/bmb/ldh034. PMID 15728132.
- ^ Albert, G. Due south.; Balamore, D. (1998). "Evolution of hyperpolarized noble gas MRI". Nuclear Instruments and Methods in Physics Enquiry A. 402 (2–3): 441–453. Bibcode:1998NIMPA.402..441A. doi:ten.1016/S0168-9002(97)00888-seven. PMID 11543065.
- ^ Ray, Sidney F. (1999). Scientific photography and applied imaging. Focal Printing. pp. 383–384. ISBN0-240-51323-i.
References [edit]
![]()
Look upward noble gas in Wiktionary, the free lexicon.
![]()
Wikibooks has more on the topic of: Noble gas
- Bennett, Peter B.; Elliott, David H. (1998). The Physiology and Medicine of Diving. SPCK Publishing. ISBN0-7020-2410-four.
- Bobrow Test Grooming Services (five December 2007). CliffsAP Chemistry. CliffsNotes. ISBN978-0-470-13500-half-dozen.
- Greenwood, N. Northward.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford:Butterworth-Heinemann. ISBN0-7506-3365-4.
- Harding, Charlie J.; Janes, Rob (2002). Elements of the P Cake. Royal Society of Chemical science. ISBN0-85404-690-9.
- Holloway, John H. (1968). Noble-Gas Chemistry . London: Methuen Publishing. ISBN0-412-21100-9.
- Mendeleev, D. (1902–1903). Osnovy Khimii (The Principles of Chemistry) (in Russian) (seventh ed.). New York, Collier.
- Ozima, Minoru; Podosek, Frank A. (2002). Element of group 0 Geochemistry. Cambridge University Press. ISBN0-521-80366-7.
- Weinhold, F.; Landis, C. (2005). Valency and bonding. Cambridge Academy Press. ISBN0-521-83128-viii.
Is Nitrogen A Noble Gas,
Source: https://en.wikipedia.org/wiki/Noble_gas
Posted by: wayswee1997.blogspot.com







0 Response to "Is Nitrogen A Noble Gas"
Post a Comment